The thermodynamic (∆H) and kinetic (kon/koff) properties of proteincarbohydrate interactions are important parameters to understand binding. The ∆H, ∆S and stoichiometry of binding can be measured using isothermal titration calorimetry (ITC) while the kon/koff , Kassoc, Kdissoc, Keq can be conveniently measured using surface plasmon resonance (SPR). SPR and ITC can also provide information on conformation changes in binding, cooperativity, ionic vs. nonionic component of binding, loss of hydrophobic domain exposure to solvent on binding, and loss of counter ions on binding events. Our group expertly uses SPR and ITC to understand the biophysics of interactions between glycans and other molecules.
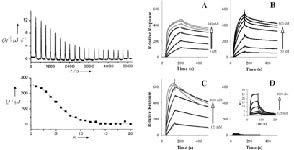
Figure. Interaction between glycosaminoglycans and proteins using ITC and SPR.
