The atomic contacts between glycosaminoglycan and its binding protein are determined by single crystal X-ray crystallography. The first high-resolution co-crystal
structure, bFGF in complex with a heparin tetrasaccharide was published by our laboratory and about half of all published co-crystal structures were done in collaboration with the Linhardt laboratory. More complex crystal structures includes the signal transduction complex, FGF2-FGFR2-(heparin decasaccharide)2 improves the understanding of growth factor signaling in the signal transduction pathway. The complex with heparin lyase bound to its heparin substrate provides an improved understanding of the mechanism and specificity of this important class of enzymes.
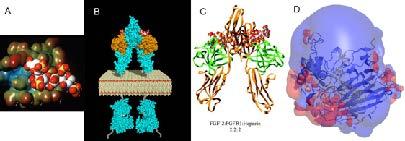
Figure. X-ray co-crystal structures. A. bFGF-heparin tetrasaccharide, B. Model of signal transduction complex, FGF2-FGFR2-(heparin decasaccharide)2 C. FGF2-FGFR2-(heparin decasaccharide)2 X-ray structure, and D. heparin lyase structure with bound heparin with surrounding charge field.
NMR solution structures of heparin-protein complexes are currently being solved of chemokines and growth factors in complex with heparin and heparin oligosaccharides.
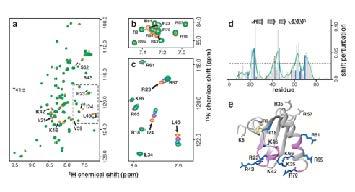
Figure. A 2D-shift map of a heparin chemokine complex showing atomic contacts between binding partners.
