The normal physiology of cells within an animal organism is regulated through signaling. This signaling can be autocrine, paracrine or endocrine, such as growth factors, chemokines, hormones, or through cell-cell contact. This complex signaling frequently involves the gycocalyx and extracellular matrix and is critical for normal physiological function. In addition to normal physiological processes, much pathophysiology is associated with the subversion of the normal glycome or an abnormal glycome. For example, in malaria, the normal glycome of the mosquito and the human hosts are subverted by the parasite to gain entry and infect. An understanding of the similarity between the mosquito and human glycome could offer novel therapeutic approaches to the prevention of this disease. In addition to malaria, GAGs are utilized in the host cell invasion of various pathogenic flaviviruses, such as Dengue virus (DENV), West Nile virus (WNV), and yellow fever virus (YFV), The initial step is to attach and concentrate on host cells and gain access to other primary surface receptors. The flavivirus then enter the host cells through a clathrin-mediated endocytosis mechanism and their envelope proteins go through conformational changes resulting in membrane fusion and release of the viral genome. All pathogenic flaviviruses unanimously utilize GAGs as their first attachment factor in their host cell invasion. For example, our previous work shows that DENV utilizes heparin sulfate as their initial attachment factor.
Zika virus (ZIKV) is a flavivirus that causes severe birth defects, however we still do not have a complete picture of their pathogenesis in host organisms. The crystal structure of ZIKV envelope protein (ZIKV E) closely resembles that of other flaviviruses, such as WNV, JEV, and DENV. There are two putative non-contiguous GAG binding regions located at similar positions in the envelope proteins of ZIKV, DENV and other flaviviruses. Examining the sequence and structure of these GAGbinding regions within the envelope proteins, in comparison with those for ZIKV E, led us to hypothesize that ZIKV E could also bind to host cell surface GAGs as an initial step in invasion. Currently we are examining binding of ZIKV E to GAG in a real time interaction study using surface plasmon resonance (SPR) to explore the role of GAGs in host cell entry of ZIKV.
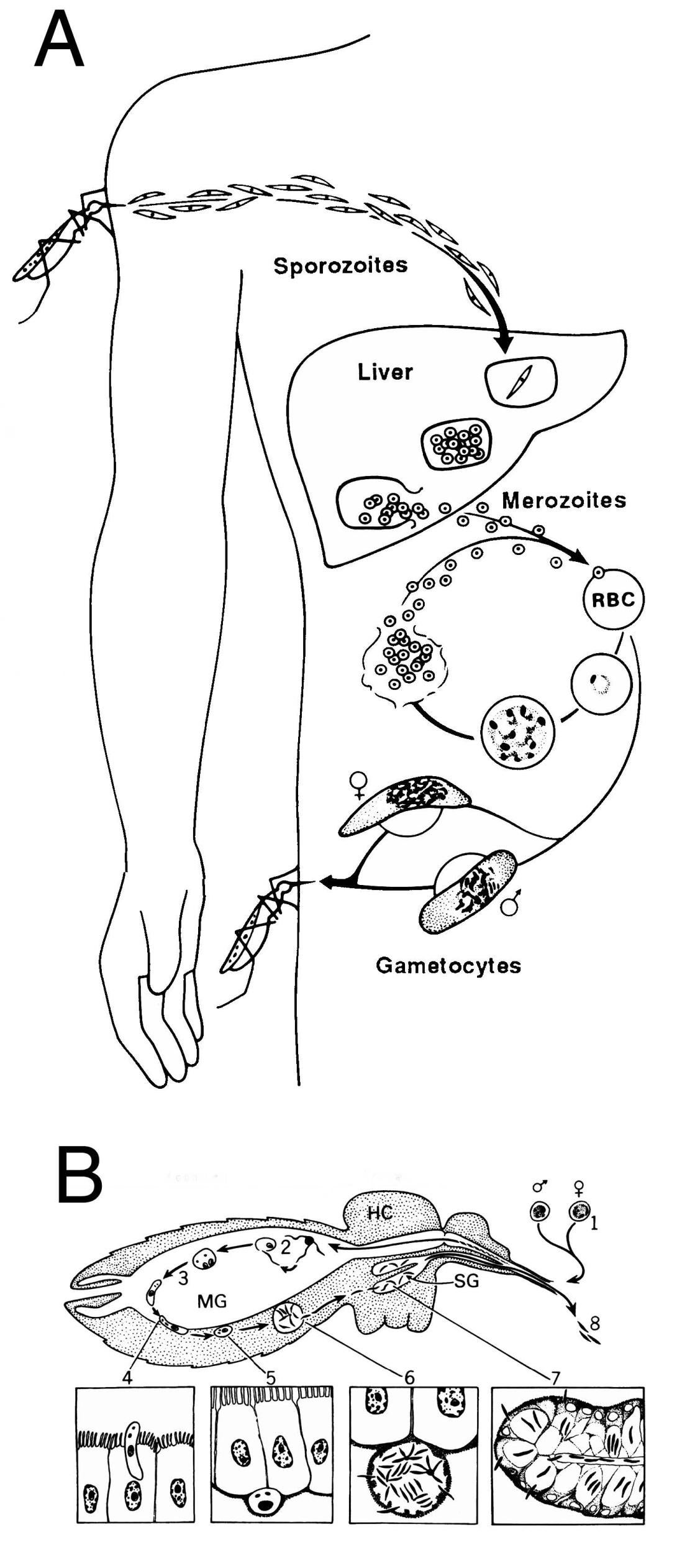
The life cycle of Plasmodium in the mammalian host (A) and the mosquito (B). A. Sporozoites are injected during blood feeding by infected Anophelene mosquitoes, go to the liver and invade hepatocytes where they divide, rupture from the hepatocyte and enter erythrocytes. The released merozoites invade erythrocytes, mature, divide, rupture from the cell and enter new erythrocytes. The asexual erythrocytic cycle gives rise to the clinical symptoms associated with malaria. Some merozoites differentiate to gametocytes, which are infective for mosquitoes. Figure reprinted with permission from Miller et al., Science 234:1349, (1986). B. Mosquitoes become infected with Plasmodium when they ingest gametocytes (1) during blood feeding. In the midgut (MG) of the mosquito, these cells differentiate (2), fertilization occurs and the resultant zygote differentiates into an ookinete (3), which traverses the midgut wall (4) and comes to rest extracellulary between the basal lamina and the midgut (5). The ookinete then differentiates into an oocyst and sporozoites develop (6), and when mature, emerge into the hemocoel (HC) of the mosquito and invade salivary glands (7;SG). When the mosquito takes another blood meal, these salivary gland sporozoites are injected into the vertebrate host (8), thus continuing the cycle.
