Unnatural UDP-sugar synthesis
Uridine diphosphate (UDP) monosaccharides are common donors being transferred to glycosyl acceptors by a glycosyltransferase or synthase in the biosynthetic pathway. Unnatural UDP-sugars, which are otherwise not synthesized in vivo, harness great potential as enzymatic substrates for carbohydrate synthesis, as enzyme inhibitors, as tools for assay development and for the study of glycoconjugate biosynthesis. Due to the prevalence of α/β 1→4 linkages throughout these GAGs, the C4 position of the UDP-donor offers an exciting target for modification. We are currently focusing on chemoenzymatic synthesis of a series of C4 modified (F, SH, NH2, N3) UDP-sugars (Gal/Glc) (Fig. 1). We found that 4F modified GlcNAc-1-phosphate and GalNAc-1-phosphate analogs could be recognized by GlmU uridyltransferase and the resulting 4F UDP-sugar do have some effect on chain length during polymerization assay. This observation herein further confirmed our previous conclusion that configuration of 4-OH group, equatorial or axial bond, appeared not to play a critical role in enzyme recognition. In addition, we discovered that even 4-halogen substituted substrates are also eligible during GlmU enzymatic reactions and could act as chain terminators for carbohydrate polymerases.
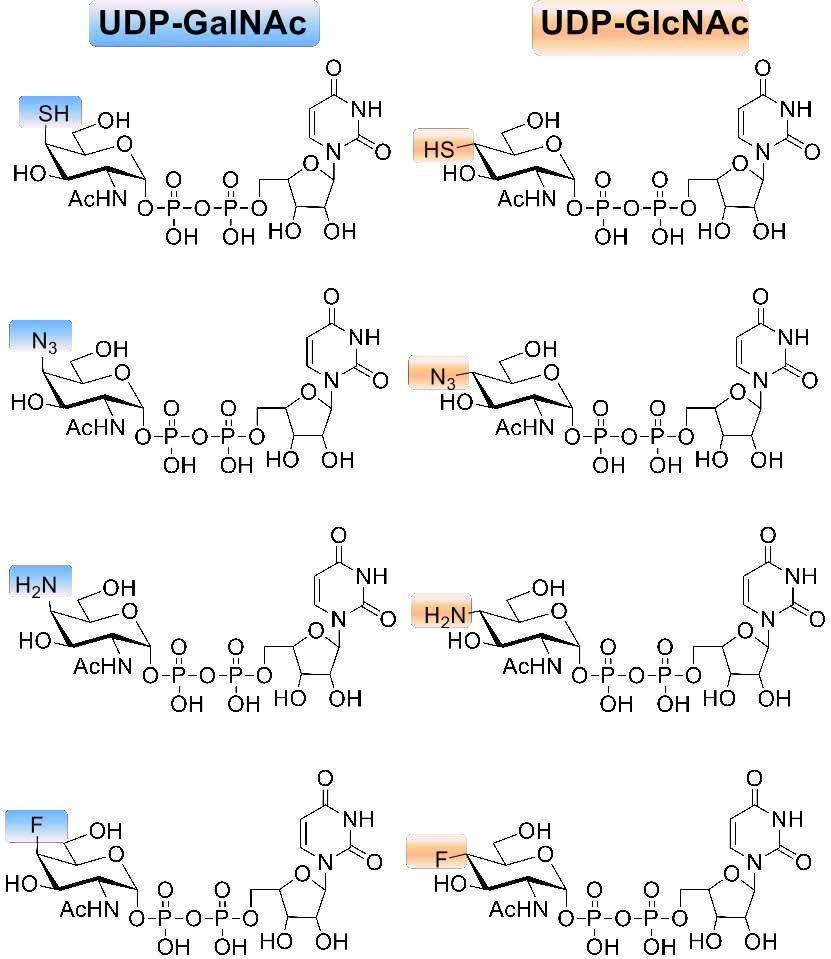
Fig. 1 Structures of unnatural UDP-sugars
